Standardization of bovine tuberculin in cattle
Abstract
ABSTRACT
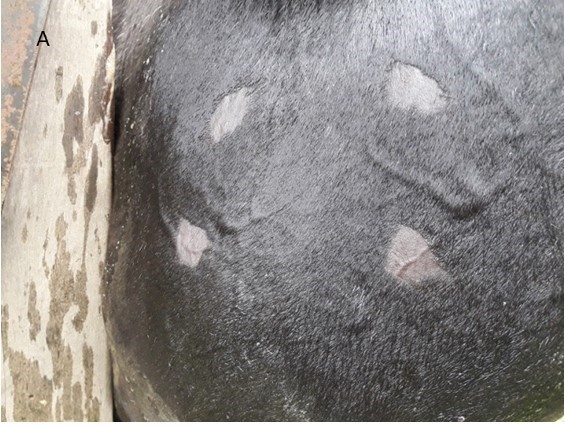
Bovine tuberculosis is a cronic infectious bacterial disease produced by Mycobacterium bovis .Infection in a live animal is diagnosed by hypersensitivity reaction delayed by intradermal immunization of bovine PPD.Laboratory control of the biological potency of Bovine PPD is carried out on nine guinea pigs sensitized with in the homologous strain.World Helath Organization (WHO) Tecnical Report Series Protocol N°384 recommends complementing these controls with tests performed on sick bovines.The objective of this study was to evaluate the biological potency and stability of PPD applied in bovines with naturally acquired tuberculosis under the conditions in which the reagent will be used .Three series of Bovine PPD tuberculinr from Ceva Animal Health were analyzed in comparison with the reference standar ( using a Latin square design ) for nine naturally tuberculous bovines and on eight inoculations sites on both sides of the animal neck.They were applied in commercial doses and 1/5 dilutions,registering the skin thicknesss reactions 72 h pos-inoculation.The reactions produced by the Ceva Bovine PPD series were higher than those produced by the standar PPD. The tuberculin potency and stability values were maintained for 13,14 and 15 months after the approval,thus concluding the stability and potency of the formulation and quality of this commercial diagnostic reagent for animal tuberculosis.
Key words : Quality control,tuberculosis bovine,tuberculin PPD,potency,stability,Argentina.
Downloads
References
Bakker, D. Good, M. (2018) Quality control of Purified Protein Derivative tuberculins: essential for effective bovine tuberculosis control and eradication programmes. In International Symposium of Veterinary Epidemiology and Economics. Chiang Mai.
Bakker, D, Eger, A, MacNair, J, Riepema, J.; Willensem, P.T.J.; Haagsma, J.; van Ziderveld, F.G.; Pollock, J.M. (2006) Comparison of commercially available PPD´s: practical considerations for diagnosis and control of bovine tuberculosis. CIDC-LELYSTAD, Department of Agriculture Rural Development,1.
Ciuca, V, Burghelea, Danes, M ,Safta, V,V, Casu, O, Niculiciolu, R, (2015) Mathematical modeling for determining the potency of bovine tuberculin purified protein derivative (P.P.D.). Medicamental Veterinar/Veterinary Drug, 9 (2): 71-75.
Daniels, W, (1991) Bioestadística. Bases para el análisis de las ciencias de la Salud. Ed. Limusa Noriega, Ciudad de México (México). ISBN 0-471-09753-5.
Duignan, A, Good, M, More, S,J, (2012) Quality control in the national bovine tuberculosis eradication programme in Ireland. Review Science Technology, 31:845-60. doi:10.20506/rs/31.3.2166, 845-860.
Duignan, A, Kenny, K, Bakker, D, Good, M.(2019) Tuberculin PPD Potency Assay in Naturally Infected Tuberculous Cattle as a Quality Control Measure in the Irish Bovine Tuberculous Erradication Programme. Frontiers in Veterinary Science, October,6 (326). doi=10.3389/fvets.2019.00328.,11-12.
European Pharmacopoeia 9.0. (2008) Tuberculin Purified Protein Derivative, Bovine 01/2008:0536,3863-3864.
Good, M, Clegg, T.A, Costello, E, Ore, S.J. (2011) The comparative performance of the single intradermal test and the single intradermal comparative tuberculosis test in Irish cattle, using tuberculin PPD combinations of differing potencies. The Veterinary Journal 190:e60-5. doi:10.1016/j.tvj1.2011.01.005.
Goodchild, A,V,Downs, S,H, Upton, P, Wood, J,L,N, de la Rua-Domenech, R. (2015) Specificity of the comparative tests for bovine tuberculosis in Great Britain. Veterinary Record, 177:258. doi:10.1136/vr.102961.
Haagsma, J, O’Reilly, L,M, Dobbelaer, R, Murphy, T,M, (1982) A comparison of the relative potency of various bovine PPD tuberculins in naturally infected tuberculous cattle. Journal Biological Standard, 10:273-84.
Maes, M., Giménez, J,F, D’Alessandro, A, de Waard, J,H, (2011) The stability of human bovine and avian tuberculin purified protein derivative (PPD) The Journal of Infection Developing Countries, 58119:781-785.
Molineri, A.I., Signorini, M.L., Pérez, L., Tarabla, H.D. (2013) Zoonoses in rural veterinarians in the central region of Argentina. Australian Journal of Rural Health, 21(5): 285-290.
Organización Mundial de Sanidad Animal (OMSA) Ex(OIE). (2018) Chapter 2.4.6. Bovine tuberculosis. In: Manual of Diagnostic tests and Vaccines for Terrestrial Animals (p.1-17.Available on line at (http://www.oie.int/standard-setting/terrestrial-manual/acces-online (accessed June 8, 2019 ) 1-17.
SENASA, Programa Nacional de Control y Erradicación de la Tuberculosis Bovina, Resolución Nº128/2012.
World Health Organization (WHO). (1987) Requirements for Biological Substances N° 16. Annex 1: Requirements for Tuberculins. Technical Report Series N° 745, WHO, Geneva, Switzerland,31-59.
Yang, H. Kruh-García, N,A, Dobos, K,M, (2012) Purified Protein Derivatives of Tuberculin-Past, Present, and Future. FEMS Immunology Medicine Microbiology, 66 (3):273-280. doi:101111/j.1574-695X.2012.01002x.
Downloads
Published
Versions
- 2024-01-05 (2)
- 2023-12-28 (1)
How to Cite
Issue
Section
License
Copyright (c) 2023 Ab Intus

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.














